Every year in low- and middle-income countries, 13 livestock-related diseases, commonly transmitted from animals to humans through water, cause 2.4 billion cases of human illness and 2.2 million deaths. Since climatic and hydrological conditions create the host environment for these disease-causing organisms, reducing the risk of infection and identifying opportunities for interventions requires a greater understanding of the connection between changing environmental and human factors and health.
‘Until now, models used to predict risk have been based on correlation between historical climate and prevalence data,’ explains Thorsten Wagener, Professor of Water and Environmental Engineering at Bristol. ‘But these don’t take account of expected changes in climate and human activity, so they’re unlikely to be reliable for understanding potential future exposure.’
This was the topic of a workshop bringing together the University’s health and environment researchers at the event co-hosted by the Elizabeth Blackwell Institute (EBI) and the Cabot Institute at the University of Bristol.
‘We realised that the rapid growth of data on human mobility, environmental conditions and human and animal health gave us the opportunity to make a step-change in how we modelled the problem,’ says Professor Wagener. ‘Specifically, it allows us to replace correlation based approaches with mechanistic models that can be used to model so-far unobserved conditions and test the potential value of interventions’. These discussions were further developed at the EBI workshop involving human and animal infectious disease modellers.
As a direct result, Professor Wagener, Professor Vickerman and Dr Morgan drew up a proposal for a cross-disciplinary project, bringing together key collaborators in the School of Social and Community Medicine, the School of Veterinary Sciences, the School of Biological Sciences, the School of Geographical Sciences, and the Queen’s School of Engineering. The team’s objective was to develop advanced infectious disease modelling that simulates current and future scenarios for waterborne diseases, allowing better planning and interventions. The project was made possible by a grant from the EBI Catalyst Fund, set up to encourage novel interdisciplinary research.
First, the team developed a conceptual model or description of the interactions between climate, human and animal mobility, hydrology, and infectious disease transmission, using a common language across the different disciplines.
Next, they combined their expertise to model these different components and develop and validate a method for assessing current and future patterns of liver fluke (Fasciola hepatica) in two UK locations. Liver fluke is a common parasite in livestock, which in the UK alone costs farmers £300 million a year due to lost production. Despite efforts to control it, UK fluke outbreaks are on the rise, and this is often attributed to climate change.
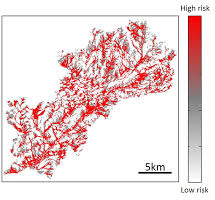
Their research led to the first integrated model for liver fluke that simulates the suitability of habitat for disease development in space and time, and the life cycle of the organism in connection with key environmental conditions. This allows a more realistic assessment of how climate change could affect the risk of future outbreaks.
The team aims to use this to secure further funding to explore the potential of the model framework across different waterborne diseases. Validation of the model is being followed by a grant application to the Wellcome Trust, supported by a research paper to be submitted to the Journal of the Royal Society Interface early 2017.
Professor Diana Williams, at the Institute of Infection and Global Health, University of Liverpool, provided the data for model testing and validation. Her team is leading liver fluke research, and this new collaboration should open up opportunities for further funding. ‘Liver fluke is causing farmers huge concern, as infection patterns are changing and making standard control protocols less useful, and as antiparasitic drug resistance takes hold’, comments Dr Morgan. ‘Our model is a unique contribution and raises Bristol’s profile in a highly fundable area. Better decision support tools for control of liver fluke certainly would improve UK agricultural competitiveness and boost food security globally.’
‘By integrating infectious disease models with detailed hydrology and climate change models, work undertaken in this project has the potential for greatly improving the use of modelling to help evaluate the health impact of interventions that have hydrological effects’, says Professor Vickerman.
A new tool for farmers has now been developed by the Bristol team to help them mitigate the risk to their livestock. The model, which works by explicitly linking liver fluke prevalence with key environmental drivers, especially soil moisture, will help farmers decide whether they avoid grazing livestock on certain pastures where liver fluke is more prevalent, or treat animals based on when risk of infection will be at its peak. Importantly, the model can be used to assess the impact of potential future climate conditions on infection levels and guide interventions to reduce future disease risk.
The five-year study comprising engineering, biology and medical researchers from the Universities of Bristol, Queen’s University, Liverpool and Scotland Rural College, was funded by the EPSRC, the Royal Society, and Bristol’s Cabot Institute and Elizabeth Blackwell Institute.