DRIFT10
The DRIFT10 study has been set up to examine the effects of surgical drainage, irrigation and fibrinolytic therapy (DRIFT) in preterm infants with post-haemorrhagic ventricular dilatation, focusing on brain function and structure at school age.
DRIFT was developed as a method of washing out the ventricles in the brain to clear the effects of bleeding. The DRIFT randomised trial was conducted in 2003-2006. The aim of the DRIFT10 study is to compare cognitive function, visual function, sensorimotor ability and emotional well-being between the children in the two treatment groups from the DRIFT trial at school age.
The study runs from September 2014 to May 2016.
Background
Despite a number of different approaches to therapy, no intervention has been objectively shown to improve outcome in post-haemorrhagic ventricular dilatation. Drainage, irrigation and fibrinolytic therapy (DRIFT) was developed as a method of washing out the ventricles to clear the effects of the bleeding.
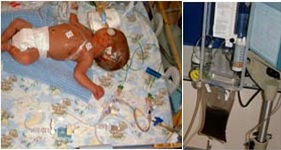
Eligible babies were preterm who had sustained intraventricular haemorrhage which expanded cerebral ventricles over pre-determined limits. With parental consent, 54 babies were randomised in Bristol and a further 20 in Poland, to either DRIFT or standard therapy which consisted of fluid tapping to control excessive expansion.
Magnetic resonance (MR) brain imaging carried out at term age in 36 babies in the DRIFT trial and showed cerebral lesions and/or impaired brain growth in a large proportion of the infants.

However, when neurodevelopment was assessed by “blinded” observers at 2 years, severe disability or death was significantly reduced in the DRIFT group. There was a large reduction in severe learning difficulties from 59% to 31% in the DRIFT group compared to the standard treatment group.
Study group
The children in the DRIFT trial are a unique study group with well-documented serious brain injury substantially different from the previous studies of preterm infants, most of whom have not had severe brain injury of this nature.
Cognitive testing at 2 years is limited, and can be difficult in children with motor impairment therefore it is important to do more comprehensive testing at school age.
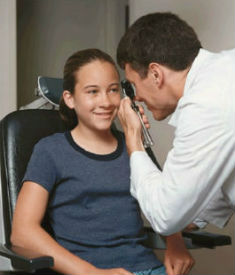 Cerebral visual impairment concerns how the brain processes images and cannot be adequately tested at 2 years. Thus it is important to thoroughly assess vision including techniques that can detect and define cerebral visual impairment.
Cerebral visual impairment concerns how the brain processes images and cannot be adequately tested at 2 years. Thus it is important to thoroughly assess vision including techniques that can detect and define cerebral visual impairment.
Combined with MR imaging, the visual and cognitive assessments at school age will allow us to assess to what extent the brain has remodelled and adapted to the previous injury and will enable us to see if some functions (i.e. vision or language) have “moved” from their normal locations.
This study may provide vital insights into how the brain repairs and adapts after injury from IVH in premature infants, enabling future refinement of treatments and might ultimately lead to a reduction in disabilities in children born prematurely, with a long-term cost saving to health and education services, and improved quality of life.
Co-investigators
Professor Andrew Whitelaw, MD, FRCPCH, Professor of Neonatal Medicine, University of Bristol and University Hospitals Bristol NHS Foundation Trust
Dr David Odd, Consultant in Neonatal Medicine, Southmead NICU, North Bristol NHS Trust
Dr Sally Jary, Research Paediatric Physiotherapist, St Michael’s NICU, UHBristol NHS Foundation Trust
Professor William Hollingworth, Professor of Health Economics, School of Social and Community Medicine, University of Bristol
Professor Pete Blair, Reader in Medical Statistics, School of Social and Community Medicine, University of Bristol
Professor Cathy Williams, MD, FRCS, Senior Lecturer in Paediatric Opthalmology, , University of Bristol, University Hospitals Bristol NHS Foundation Trust and North Bristol NHS Trust
Dr Michelle Morgan PhD, Clinical Child Psychologist, North Bristol NHS Trust
Dr Grazyna Kmita, Clinical Child Psychologist, Faculty of Psychology, University of Warsaw
Mr Ian Pople, Consultant Paediatric Neurosurgeon, North Bristol NHS Trust
Mr Kristian Aquilina, Consultant Paediatric Neurosurgeon, Great Ormond Street Hospital for Children NHS Trust
Mr Steve Walker-Cox, PPI Advisor and Parent.


