FITNET-NHS: Frequently Asked Questions
Please note, as of 11 November, we are no longer recruiting new participants into the FITNET-NHS Trial, though treatment is still available. See here for details:
~Frequently asked questions about FITNET-NHS in the wake of COVID-19~
-About CFS/ME-
Q. What is CFS/ME?
Q. How do you know whether someone actually has CFS/ME?
-Why is this study important?-
CFS/ME is common. At least 1% of teenagers miss a day a week of school because of CFS/ME. Children and young people with CFS/ME suffer because they cannot do what they want to do. They miss large amounts of school, social activities and sport. We know that mothers often have to reduce or stop work (and we assume this is likely to be true for all care givers). The National Institute of Health and Clinical Excellence (NICE) says that children with CFS/ME should be able to access specialist care and treatment. Specialist treatment is effective but most children in the UK do not have a local specialist service and therefore cannot access treatment.
We need to improve treatment for children and young people who have CFS/ME but do not have access to a local specialist CFS/ME service. In this study we will test two treatments that can be delivered at home. The FITNET-NHS study provides two interventions: either cognitive behavioural therapy (CBT) online or Activity Management delivered via video call (Skype).
FITNET was effective in the Netherlands [FITNET Lancet 2012 (PDF, 143kB)]. The Dutch study was different to this study as in the Dutch study, participants who did not receive FITNET only received usual care. In our study, children and young people will receive either FITNET-NHS or Activity Management. We don't know which treatment patients will prefer or which is better for them. We are doing this study because we need to know which treatment is best. We also need to know whether treatment is good value for money before the NHS can use it. Activity Management is used as the comparator in this study as it is recommended by NICE and is currently the best alternative for children in regions without a local specialist CFS/ME service.
About 30% of children and young people with CFS/ME develop problems with low mood or anxiety. We do not know whether treatment is effective for this group of patients. Our study will be able to tell us whether FITNET-NHS is effective for children and young people with CFS/ME and low mood or anxiety.
-Treatment within the study-
Q. What happens in the study?
A. Children will be randomised to receive either Activity Management or FITNET-NHS.
Both treatments will: give advice on sleep and building up physical, thinking and school activities, have one to one contact with a therapist and give advice based on your individual symptoms, activity levels and goals. This issimilar to what is provided in face to face treatment in Bath.
Those who get Activity Management will receive information on managing activities and sleep from a CFS/ME specialist therapist over up to six video (Skype) calls. Therapists will provide a detailed assessment of each participant’s daily activities and will give advice and support on how to increase this level safely over time. Children will be asked to do homework (complete diaries via the ActiveME App). The specialist therapist will hand over care to the local GP or paediatrician but will provide support to them with up to three phone calls.
Those who receive FITNET-NHS (and their parents) will complete an online Cognitive Behavioural Therapy (CBT) type treatment developed specifically for children with CFS/ME. The 19 interactive online chapters cover sleep, building up physical, mental (including school), and social activities and thinking patterns. . Children will be asked to do homework (answer questions and complete diaries). Each child and parent will be in contact with their allocated CBT-trained therapist over email and the therapist will review progress according to individual goals and support behaviour change.
Q. How is treatment in the trial different to what I would receive through usual care in the Bath Specialist Paediatric CFS/ME service?
A.Therapists providing treatment through the FITNET-NHS trial are the same therapists that you would see face to face in Bath. The treatments on the FITNET-NHS trial are very similar to what is already provided in face to face care in Bath. Both treatments will: give advice on sleep and building up physical, thinking and school activities, you will have one to one contact with a therapist that will give advice based on your individual symptoms, activity levels and goals.
The difference is that the FITNET-NHS trial is looking to see whether providing treatment to children with CFS/ME at home works. We know that many patients with CFS/ME get find travelling difficult and may experience an increase in symptoms. In Activity Management, you and your parent(s) will talk to a therapist over Skype. In FITNET-NHS, you and your parent(s) will talk to a therapist over emails and complete treatment chapters online. FITNET-NHS also includes chapters focusing on CBT, helpful thoughts and shifting attention away from fatigue.
Q. What are the differences between the treatments in the trial?
A. The treatments are actually very similar. Both treatments give advice on sleep and building up physical, thinking and school activities, have one to one contact with a therapist that will give advice based on individual symptoms, activity levels and goals.
The main differences are in the ways the treatment is delivered. Activity Management is delivered by six video (Skype) calls with your allocated therapist. FITNET-NHS is delivered through online modules as well as one to one emails your allocated therapist. FITNET-NHS also includes chapters focusing on CBT, helpful thoughts and shifting attention away from fatigue. There are more treatment modules in FITNET-NHS but we do not know if this is helpful or not.
Q. What is Cognitive Behavioural Therapy and why is this being used to treat Chronic Fatigue Syndrome?
A. Cognitive Behavioural Therapy (CBT) is recommended by the NICE Guidelines for CFS/ME (2007) due to many studies in both adults and adolescents indicating that CBT is a helpful intervention for CFS/ME. You can read about the Dutch FITNET trial below in this FAQs list.
CBT explains that there is a connection between what you think, feel and do. Sometimes it is hard to change what we do. By identifying helpful thoughts and behaviours you can have a positive impact on what you do and how you feel.
CBT is used to improve people’s quality of life in a range of other health conditions such as diabetes, multiple sclerosis and cancer care. Through FITNET-NHS we help you to think about the current ways you manage this physical illness and think together about helpful ways forward to enable you to reach your goals. By making changes to what you do, you can change the biological processes in your body, which can help you to recover.
Q. Will I have contact with a therapist if I take part in the trial?
A. Yes. You will be assigned to an individual specialist paediatric CFS/ME therapist that works at Bath. In activity management you will talk to your therapist via six video (Skype) calls. On FITNET-NHS you will talk to your therapist via emails (average 28 emails over 6 months).
Q. Will treatments in the trial involve a lot of work?
A. For both treatments you will be asked to complete diaries either online (FITNET) or through the ActiveME App (Activity Management). This helps the therapist assess your current level of activity to set a manageable starting point (baseline level) and gradually build up. It will also help children and young people taking part in the study to see their progress as time goes on.
In activity management you will have to attend Skype calls with a therapist. In FITNET-NHS you will have to read chapters and answer questions. There is a lot of information at the start of treatment but patients have reported that this gets easier at time goes on.
Q. Will treatments involve exercise?
A. Both treatments will help you manage your activity better. Activity can include sleep, physical activity (e.g. walking) and mental activity (e.g. schoolwork). The advice you will receive will depend on the exercise you are doing, the pattern of exercise and what you want to achieve. For most children this will involve stopping doing a lot of exercise which can increase your symptoms and help you set a manageable level to start at and gradually build up at safe amount.
-How to access the study - and who can take part-
Q. How can children and young people access this study?
A. Children and young people need to be referred to the Bath Specialist Paediatric CFS/ME Service by their GP. To be eligible for this study, they need to have been assessed by a Paediatrician, have had their screening blood tests done (see NICE guidance for more information). This is to make sure other causes of fatigue have been excluded.
Q. Can you take part if you are severely affected by CFS/ME?
A. Yes, you can take part if you are severely affected. The therapists with adapt the treatment on the trial to suit your level of functioning.
Q. Do parents take part in treatment as part of the trial?
A. Parents take part in the video (Skype) calls as part of Activity Management. However, if a child/young person or parent(s) want to talk to a therapist on their own at any point they can ask. In FITNET-NHS parents also have online modules to read through. They will also be individually emailing the therapist as well as their child or young person.
Q. Will children and young people with co-morbid conditions (such as diabetes) be able to take part in the study?
Q. How many children and young people will be recruited to the study with post-exertional malaise?
Q. Will children without post exertional malaise be recruited?
A. Post exertional malaise is what we consider to be a defining symptom. Checking that children have post exertional malaise is part of our recruitment pathway. More details of this can be found in the protocol.
Q. Can patients from Scotland, Wales and Northern Ireland enter the FITNET-NHS study?
Q. Can I take part in the study if I am an adult with CFS/ME (18 years and older)?
A. The study is only for children aged 11-17 years old. However, there are a number of specialist CFS/ME services for adults: ME Association
Q. Can I take part in the study if I have left school and I am working?
- Duration of the study and questionnaires -
Q. How long will I be in the study?
A. Treatment in both arms is designed to take around 6 months to complete. You and your parents/carers will be asked to complete questionnaires at the start (baseline), 3 months, 6 months and 12 months which will take you about 20 minutes each time. So even if you are finished treatment, we will still ask you to complete a questionnaire at 12 months to see how you are getting on and if treatment is working/ worked. This is how we can tell which treatment is best.
Q. How long will this study take?
A. The project was funded from May 2016 and opened to recruitment on 1 November 2016. We hope to publish the results in May 2022 but it will depend on how long it takes us to recruit 314 children. We have estimated that this may take 42 months.
-Results of the study-
Q. Why does this have to be such a large study?
A. We are hoping to recruit 734 children and young adults. About 30% of children with CFS/ME also have low mood or anxiety. This large sample size will allow us to know if this treatment will work for those with CFS/ME and depression or anxiety. This is an important group of patients for whom there is little treatment available.
Q. What will happen at the end of the study?
A. If FITNET-NHS is effective and is good value for money, we will be able to continue delivering FITNET-NHS to patients at the end of the trial. There is little roll out involved because it can be delivered from the same trust by the same therapists.
Q. What will happen to the results from this study?
A. The FITNET-NHS study will be published in the HTA Journal part of the NIHR Journals Library in 2022, providing an important and permanent record for research funded by the National Institute for Health Research. All of the journals are freely available to search, view and download from the NIHR Journals Library website.
NIHR report authors are expected to publish results in other high-quality peer-reviewed journals as part of their dissemination strategy. The NIHR has had a policy on open access since 2006.
-Previous research - the Dutch FITNET Trial-
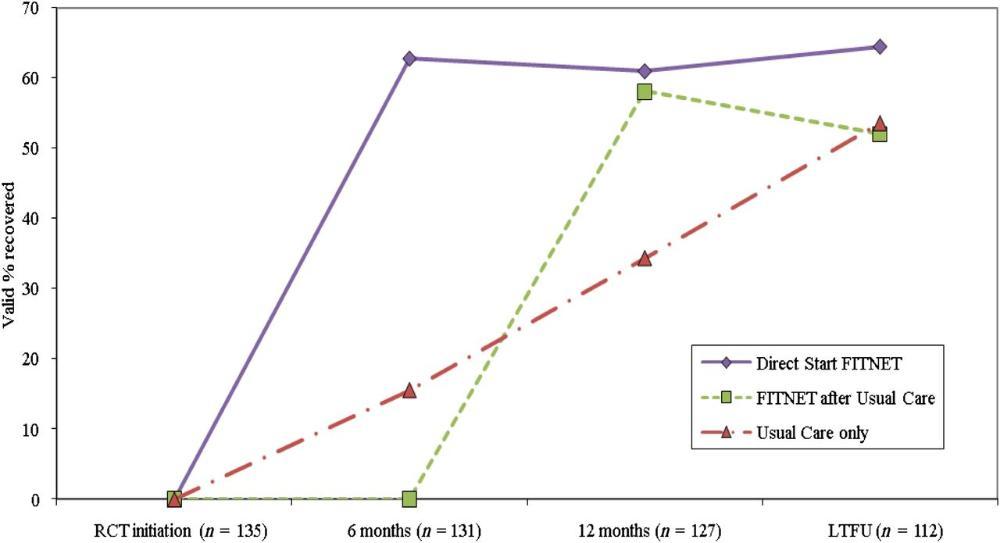
Q. What did the Dutch FITNET trial show?
Q. But didn't the Dutch teenagers recover anyway, whether or not they received FITNET?
Q. So what difference did FITNET make to Dutch teenagers with CFS/ME?
-Research Funding-
Q. Who is funding the research?
A. The study is funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (14/192/109). The study proposal was submitted through the NIHR HTA Programme’s researcher-led workstream (http://www.nets.nihr.ac.uk/programmes/hta/funding-streams) by Dr Esther Crawley.
Q. Why has this research been funded?
A. Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) affects 1-2% of young people, most of whom do not have access to treatment. This research is investigating whether FITNET-NHS is effective and cost effective. This is because we need to be able to offer treatment to children and young people with CFS/ME who cannot access local specialist treatment. If FITNET-NHS is effective and cost-effective, we will able to offer treatment for children throughout the NHS wherever they live.
Q. What is the process for commissioning research?
A. The NIHR HTA Programme researcher-led work stream funds research questions proposed directly by researchers. Applications are usually assessed using a two stage process. All research proposals are assessed at each stage by the HTA Board whose members are appointed for their expertise. External expert opinion is also obtained from a number of national experts in the field.
The HTA Programme funds independent research about the effectiveness, costs and broader impact of healthcare treatments and tests for those who plan, provide or receive care in the NHS. The board looks at whether the study is designed to achieve its objectives in an appropriate, feasible and ethical manner, whether successful completion of the project would lead to a reduction in uncertainty and whether the research would result in measurable health gains for patients and/or wider benefits for the NHS. They will also judge whether the proposal is methodologically and scientifically robust and if the team expected to carry out the research have the necessary skills, experience, project management and infrastructure for success.
Q. Is FITNET-NHS being done to save the NHS money?
A: We don’t know if FITNET-NHS will save money in the long run. We may find that it is more expensive than usual care, as the treatment involves lots of therapist contact. One of our questions we are looking to answer with this research is finding out what the treatment costs (on average) and whether it is cost effective as a treatment for CFS/ME.
Q. What level of funding has been provided for this study?
A. The HTA as agreed to fund this study at a cost of £994,430.
