If you need to make any change to a study over the course of its conduct, this is referred to as an Amendment. This could be, for example, a change to protocol procedures, to participant-facing documents, to key study-staff, to the sites conducting the study, etc.
For any such change you will need to complete the Amendment Tool. You can find the current version of the tool in the IRAS Help pages. This may be updated without prior warning and you are expected to submit using the current version,so please download from IRAS each time, rather than saving a local version.
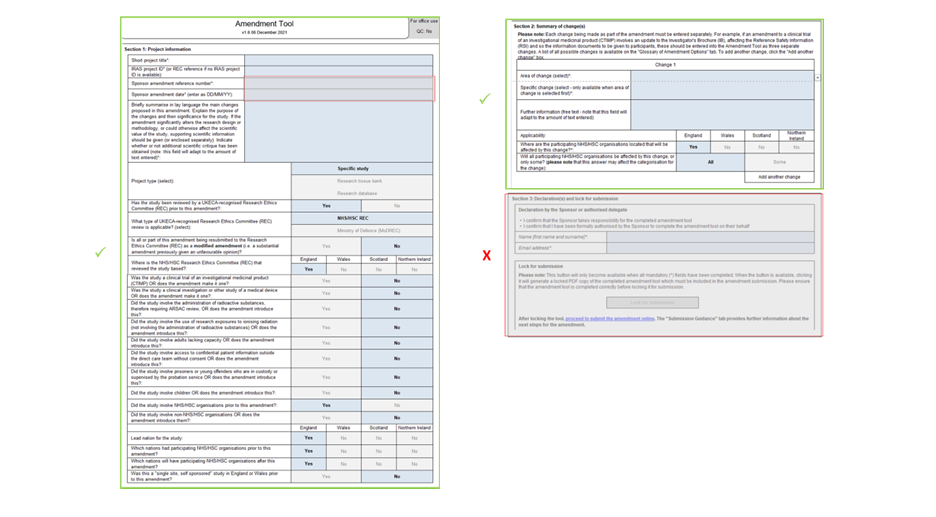
Once you have selected the appropriate categories to describe the nature of your amendment, the tool will determine for each of the bodies that approved your study whether this is a Substantial Amendment, requiring full review; a Non-Substantial Amendment, requiring formal submission and acknowledgement; or a non-Notifiable Amendment, which will be managed by your sponsor.
Categories will be checked as part of Sponsor Review, to ensure that they are the most accurate available descriptions of the intended changes.
It is a requirement that the Sponsor organisation review Amendments prior to their being submitted. Once you have drafted the Amendment Tool, please send it to research-governance@bristol.ac.uk, in its original Excel format.
Leave lines 8 & 9 and all of Section 3 blank, these will be completed by the Sponsor.
Once the form has been reviewed and agreed, it will be returned to you as a pdf. It should then be submitted via the IRAS amendments system, for which you will need to create an account, in addition to your regular IRAS account.