Podocytes
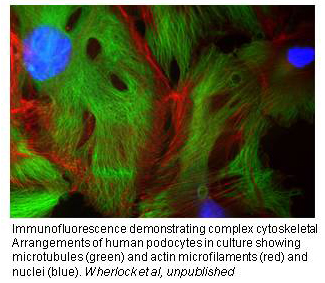
Podocytes have a remarkably elaborate and highly specialised morphology that is dependent on the actin cytoskeleton and which is essential for maintaining glomerular function and integrity in healthy kidneys. There is also now compelling evidence that podocytes display a limited physiological motility, that would be important for example in unclogging of the filter, and a growing appreciation that changes in podocyte motility may underlie nephrotic disease. Thus it is clear that the specialized function of the podocyte in the normal kidney depends critically on an underlying network of dynamic and interconnected actin and microtubule polymers. The mechanism through which this morphology is achieved and maintained in the normal kidney is not currently understood. However, the recent discovery that a number of human steroid resistant nephrotic syndromes (SRNS) are caused by genetic mutations coding for podocyte-specific proteins or proteins that seem only dysfunctional within the podocyte provide important clues as to the nature of key players in this process. Indeed all of the proteins discovered so far are associated with the slit diaphragm or directly link it to the podocyte actin cytoskeleton.
It has been ascertained that the glomerular podocyte is the first cell to get damaged in SRNS. Using our wild type and ‘natural knock-out’ podocyte cell lines and human SRNS patient plasma samples in the laboratory has enabled us to identify putative circulating factors in plasma that may be causing both the primary and recurrent forms of the disease – a holy grail of research in kidney disease. This hypothesis needs to be tested in a significantly expanded cohort of patients, in a consistent and cohesive manner, and this project aims to address this problem. These advances have brought us to the brink of translational applications that would transform the management of a disease that has remained an enigma for decades.
Because of the rarity of SRNS , the incidence of new patients in the UK is relatively small. We have established a new national cohort, collecting detailed phenotypic and genotypic data on all incident patients in the UK, as well as plasma samples peri-transplantation. This cohort is the pilot in a new national strategy to coordinate clinical care, management and research into renal rare diseases.
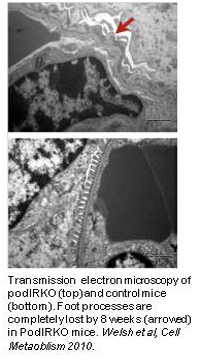
We were first to report that human podocytes take up glucose in response to insulin in a manner similar to metabolic storage cells such as myocytes. Other renal cells, including GEnC, show no such response. Cellular insulin resistance is a key event in the development of metabolic syndrome and diabetes, the most important causes of kidney failure world-wide. Using our human cell lines and a transgenic mouse that had its insulin receptor specifically deleted in the podocyte (podIRKO mouse), we have recently shown that insulin sensitivity of the podocyte is crucial for glomerular function and that without it animals develop significant albuminuria together with histological features that recapitulate diabetic nephropathy, but in a normoglycemic environment. We are currently investigating the role of insulin like growth factors in this phenomenon.
Genome-wide association studies in 3 separate Caucasoid populations were undertaken to precisely delineate the genetics of idiopathic membranous nephropathy. We showed tight linkage with two genetic regions: an immune response gene in the HLA-DQA1 region, and a region encoding phospholipase A2 receptor (PLA2R), recently identified as the predominant autoantigen in IMN 59. The relative risk for development of IMN in the presence of the risk alleles at these two loci was an extraordinary 78.5 (p = 10-93). PLA2R is known to be expressed by podocytes but the genetic association study does not prove that it is podocyte PLA2R that is important in the causation of the disease. We are currently testing this hypothesis.
