What Are Surfactants
The term colloid describes a system consisting of one substance finely dispersed in another, normally a solvent. The size of particles found in a colloidal dispersion range between 1 and 1000 nanometres. The two components are referred to as the dispersed phase and continuous phase respectively. Either phase can be a solid, liquid, or gas, creating a range of possible colloidal systems. Many colloids exist in nature, whilst others are man-made, examples of both include:
- Milk (liquid fat dispersed as fine drops in an aqueous continuous phase)
- Smoke (solid particles dispersed in air)
- Bone (small particles of calcium phosphate dispersed in a solid matrix of collagen)
- Paint (small solid particles dispersed in a solvent)
- Shaving foam (tiny air bubbles dispersed throughout a liquid soap)
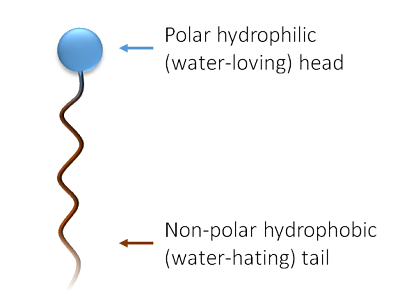
Figure showing the general structure of a surfactant molecule
Generally speaking, molecules may be considered as either polar or non-polar. Polar molecules will readily mix with other polar molecules, but not so well with other non-polar molecules (and vice versa). An easily visualised example of this is water, a polar liquid, mixing poorly with oil, a non-polar liquid. If a molecule is polar it is hydrophilic ("water-loving"), whereas if a molecule posses no polarity then it may be hydrophobic (“water-hating” or also "oil-loving"). Surfactants, a contraction of the term surface-active agents, are amphiphilic (dual-natured) molecules because they possess both hydrophilic and hydrophobic groups. The general structure of a surfactant molecule is shown in the figure right. A surfactant molecule posses both a polar “water-loving” headgroup attached to a non-polar “water-hating” (or “oil-loving”) tail.
Due to their dual nature, they are associated with many useful interfacial phenomena, and as such are key components for many diverse industrial products and processes.
