
Professor Jonathan Hanley
B.A.(Cantab.), Ph.D.(U.C.Lond.)
Current positions
Professor of Molecular Neuroscience
School of Biochemistry
Contact
Press and media
Many of our academics speak to the media as experts in their field of research. If you are a journalist, please contact the University’s Media and PR Team:
Research interests
Jonathan Hanley, Professor of Molecular Neuroscience
Learning and the formation of memories involves alterations in neural circuitry, brought about by changes in synaptic strength, which are in turn underpinned by changes in the molecular machinery of synapses. We use a range of biochemical, molecular and imaging techniques, and we collaborate with electrophysiologists for analysing synaptic function. We study three aspects of synaptic plasticity: AMPA receptor trafficking, dendritic spine morphogenesis and the control of protein translation by microRNAs.
AMPA receptors (AMPARs) mediate the majority of fast excitatory synaptic transmission in the brain, and plasticity at excitatory synapses involves alterations in the number of AMPARs localised at the synaptic plasma membrane, brought about by regulated receptor trafficking. AMPAR expression at the synaptic plasma membrane is regulated by endocytosis, exocytosis, recycling and lateral diffusion events that contribute to reductions (Long Term Depression, LTD) or increases (Long Term Potentiation, LTP) in synaptic strength.
A major focus of the lab is investigating how basic cell biological processes interact with AMPAR subunits to bring about changes in AMPAR trafficking and hence synaptic strength. In particular, we are studying a PDZ- and BAR-domain protein called PICK1, which binds AMPAR subunit GluA2, and is involved in AMPAR internalisation and LTD. We have demonstrated that PICK1 inhibits Arp2/3-mediated actin polymerisation, and that this is required for NMDA-stimulated AMPAR internalisation and LTD (Rocca et al., 2008, Nat. Cell Biol; Nakamura et al., 2011, EMBO J.; Rocca et al., 2013, Neuron). We are also studying how PICK1 interacts with core components of the endocytic machinery to regulate clathrin-coated vesicle formation (Fiuza et al., 2017, J. Cell Biol.).
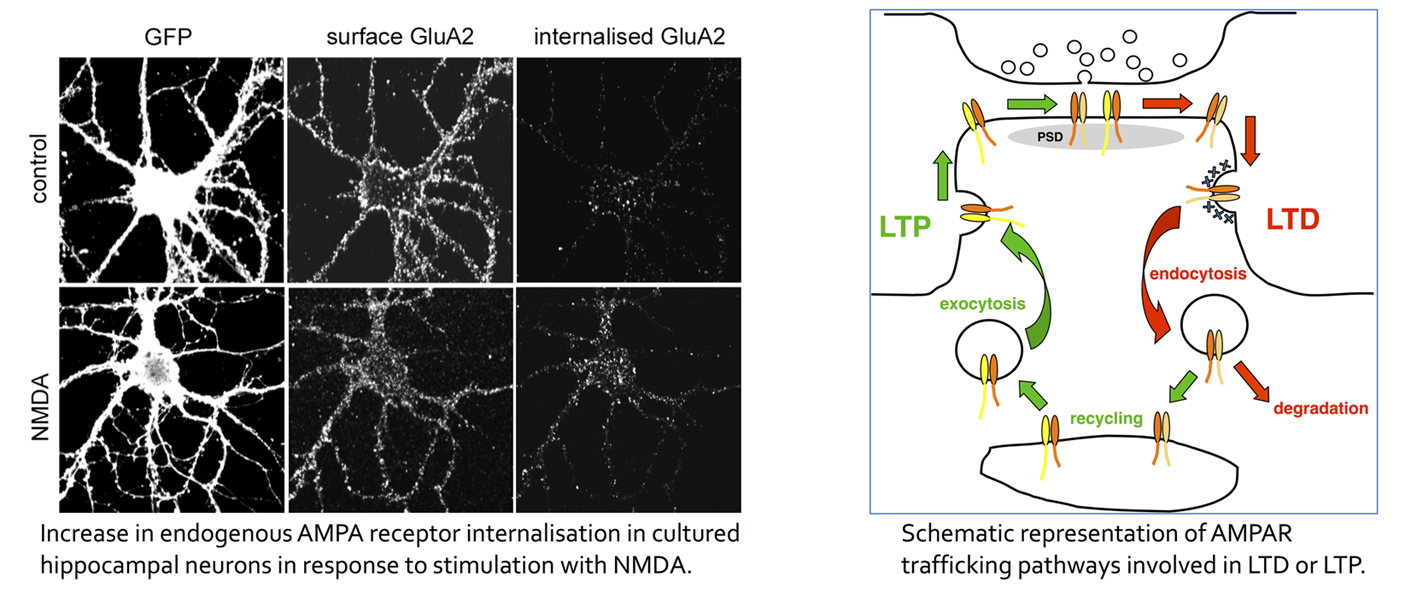
As well as “normal” plasticity, we are studying AMPAR trafficking events that occur in response to acute injury, such as mechanical injury (a model for traumatic brain injury, TBI) and oxygen/glucose deprivation (OGD; a model for ischaemia). Brain ischaemia occurs when the blood supply to the brain is interrupted, for example by occlusion following a stroke, or as a result of cardiac arrest. We are studying the specific endosomal sorting steps that are involved in regulating cell-surface AMPARs in response to OGD or TBI. The majority of AMPARs contain GluA2 subunit, and consequently are Ca2+ impermeable. OGD and TBI cause trafficking events that result in the loss of surface GluA2-containing AMPARs, and therefore an increase in Ca2+ permeable AMPARs, which contribute to neuronal death. We have discovered differences in subunit-specific AMPAR trafficking events between hippocampal and cortical neurons that we propose play a role in the differential vulnerabilities to ischaemia between these neuronal types observed in vivo (Blanco-Suarez et al., 2014, J. Biol. Chem; Koszegi et al., 2017, Scientific Reports).
Dendritic spines are highly specialized subcellular compartments that contain the postsynaptic protein machinery of most excitatory synapses. They concentrate and compartmentalise biochemical signals such as Ca2+, and synaptic protein machinery such as neurotransmitter receptors, providing the synaptic specificity required for plasticity. Changes in synaptic strength correlate with corresponding changes in dendritic spine size, and possibly shape. LTD stimuli result in spine shrinkage and retraction, whereas LTP leads to the formation of new spines, or the growth of existing ones.
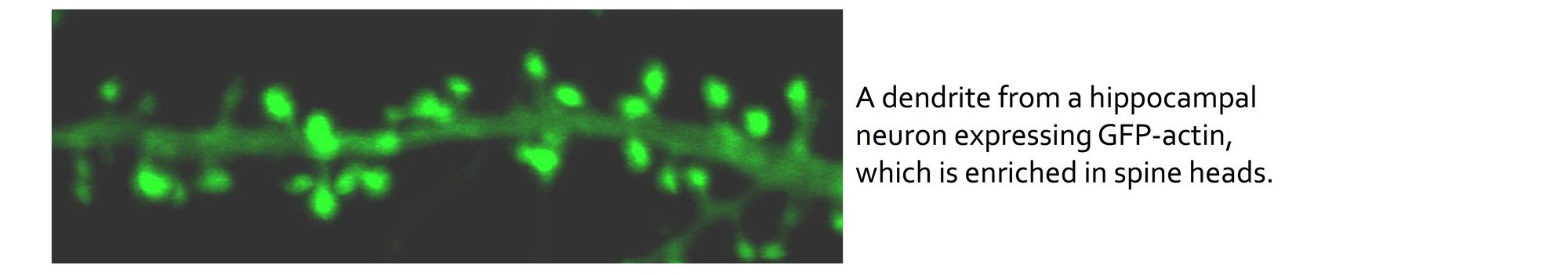
We are studying the molecular mechanisms that underlie changes in spine size during synaptic plasticity (Nakamura et al., 2011, EMBO J.; Rocca et al., 2013, Neuron) and also in response to OGD (Blanco Suarez et al., 2014, J.Cereb. Blood Flow Metab.).
Spines are extremely rich in dynamic actin filaments, which underlie these activity-dependent changes in spine size. LTP is thought to promote actin polymerization resulting in an increase in spine F-actin, and LTD results in reduced F-actin via actin depolymerization. The signalling pathways that lead to these changes in actin polymerization to bring about spine growth or shrinkage are affected by OGD, and we prose that manipulating these pathways holds therapeutic potential.
Long-term changes in synaptic efficacy that underlie the persistent formation of memories require changes in the synthesis of synaptic proteins by the activity-dependent regulation of local translation of mRNA in dendrites close to synapses. A role for microRNAs (miRNAs) in this process has recently become the subject of much attention. MiRNAs are small, noncoding endogenous RNA molecules that repress the translation of target mRNAs through complementary binding in the transcript 3’-untranslated region (3’-UTR). A number of miRNAs have been shown to be involved in specific forms of learning and memory, and dysfunction of miRNAs is implicated in neurological and neuropsychiatric diseases including Alzheimer’s, Huntington’s and Schizophrenia.
Argonaute (Ago) proteins associate with miRNAs as well as numerous additional proteins in RNA-induced silencing complexes (RISCs) to direct translational repression of target mRNAs. We are investigating how the induction of synaptic plasticity affects this process, by studying the dynamics of RISC protein-protein interactions, how they are regulated (phosphorylation, direct binding of Ca2+, etc.) and their impact on synaptic structure and function. In a current project, we have found that the interaction between Ago2 and key RISC components including GW182 are increased by Ago2 phosphorylation in response to the induction of LTD. This mechanism leads to increased translational repression of key proteins involved in synaptic structure, and hence causes spine shrinkage (see diagram; Rajgor et al, 2018, EMBO Journal).
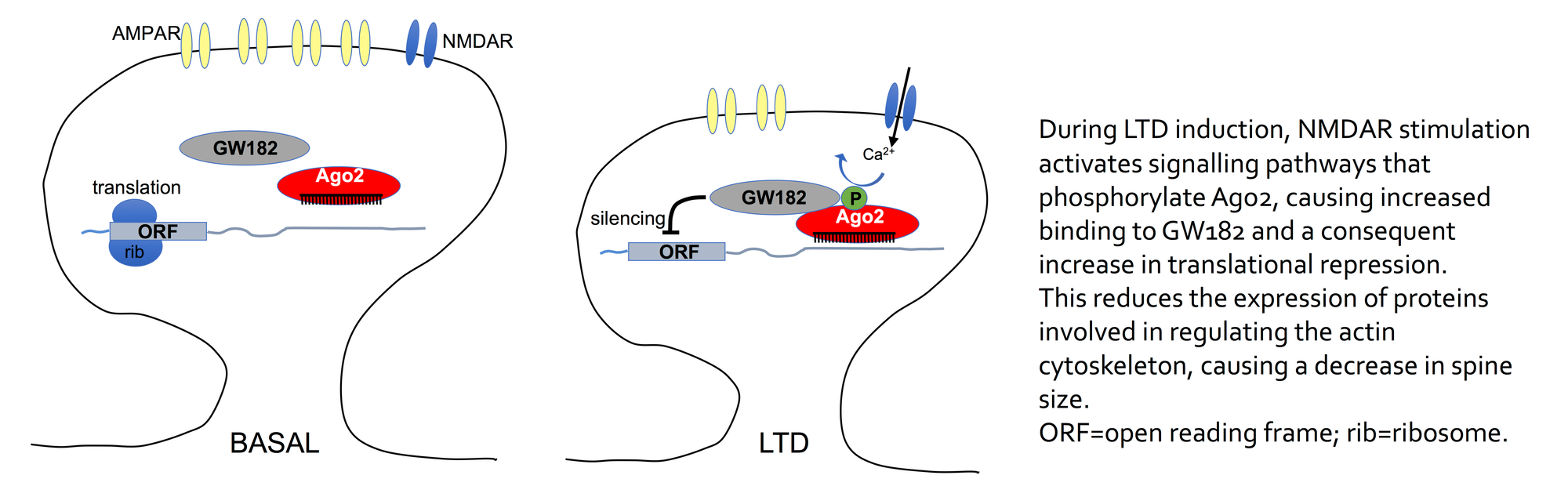
We have also demonstrated that PICK1 inhibits miRNA activity by binding to Ago2 on endosomal compartments in dendrites (Antoniou et al., 2014, EMBO Reports). This interaction is disrupted by Ca2+ ions, and therefore NMDAR-dependent LTD causes a dissociation of Ago2 from PICK1, increasing miRNA activity and hence translational repression (Rajgor et al., 2017, J.Biol.Chem).
We are also very interested in how these mechanisms of RISC regulation are affected by pathologies such as Alzheimer's and ischaemia.
Group members
Georgiana Stan, Aidan Kenny, Jessica Walters, Fathima Murshida, Sofia Raak, Kate Pring
Projects and supervisions
Research projects
Rapid silencing of specific populations of genes for neuronal plasticity and memory
Principal Investigator
Managing organisational unit
School of BiochemistryDates
01/06/2025 to 31/05/2028
Rapid silencing of specific populations of genes for neuronal plasticity and memory
Principal Investigator
Managing organisational unit
School of BiochemistryDates
01/06/2025 to 31/05/2028
Impairment of neural plasticity and adaptive representations by genetic risk factors for schizophrenia
Principal Investigator
Role
Principal Investigator
Managing organisational unit
School of Physiology, Pharmacology & NeuroscienceDates
01/10/2023 to 30/09/2028
Regulation of microRNA-mediated local translation in neurons by Argonaute phosphorylation
Principal Investigator
Managing organisational unit
School of BiochemistryDates
04/07/2018 to 03/01/2022
PICK1 and cortactin as antagonistic regulators of Arp2/3-mediated actin polymerisation in GluA2-dependent AMPA receptor trafficking
Principal Investigator
Managing organisational unit
School of BiochemistryDates
23/06/2014 to 22/06/2017
Thesis supervisions
The dynamic regulation of PICK1 BAR domain dimerisation during synaptic plasticity
Supervisors
Investigating DDX6 and its potential role in miRNA-mediated gene silencing events in neurones.
Supervisors
Investigating the extranuclear roles of the SUMO protease SENP3 in neurons
Supervisors
Ago2/DDX6-Dependent miRNA Activity in Neuronal Plasticity
Supervisors
Understanding the specificity and mechanism of gene silencing by Argonaute2 phosphorylation in Neurons.
Supervisors
Local control of synaptic protein synthesis in neurons by microRNAs
Supervisors
Resolution of the interaction site within 4R0N-tau mediating augmentation of L-type calcium current
Supervisors
Investigating mitochondrial fission factor (Mff) SUMOylation and effects on ubiquitination
Supervisors
The Lysosomal Targeting of AMPARs in Response to Amyloidopathy
Supervisors
Publications
Selected publications
24/07/2013The Small GTPase Arf1 Modulates Arp2/3-Mediated Actin Polymerization via PICK1 to Regulate Synaptic Plasticity
Neuron
The antagonistic modulation of Arp2/3 activity by N-WASP/WAVE2 and PICK1 defines dynamic changes in astrocyte morphology
Journal of Cell Science
PICK1 inhibition of the Arp2/3 complex controls dendritic spine size and synaptic plasticity
EMBO Journal
Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis
Nature Cell Biology
PICK1 Mediates Transient Synaptic Expression of GluA2-Lacking AMPA Receptors during Glycine-Induced AMPA Receptor Trafficking.
Journal of Neuroscience
Recent publications
19/02/2025Competition effects regulating the composition of the microRNA pool
Journal of the Royal Society Interface
An essential role for the RNA helicase DDX6 in NMDA receptor-dependent gene silencing and dendritic spine shrinkage
Scientific Reports
Ca 2+ Regulates Dimerization of the BAR Domain Protein PICK1 and Consequent Membrane Curvature
Frontiers in Molecular Neuroscience
Tau isoform-specific enhancement of L-type calcium current and augmentation of afterhyperpolarization in rat hippocampal neurons
Scientific Reports
c-Abl regulates a synaptic plasticity-related transcriptional program involved in memory and learning
Progress in Neurobiology


