Genetic mapping improves understanding of osteoarthritis
Using multi-disciplinary techniques to map the development of osteoarthritis in zebrafish genes allows a greater understanding of joint deterioration in humans.
It is one of the UK’s major causes of disability, affecting eight million people. Over half the population of the Western world aged over 65 are affected by it. Yet osteoarthritis, and particularly the prevention of it, attracts a proportionately low level of research interest. For Dr Chrissy Hammond, who started her Arthritis Research UK fellowship in Bristol in 2010, this makes it an increasingly urgent issue, and her research is already making significant inroads into our understanding of joint deterioration.
Dr Hammond’s approach is refreshingly novel – from the animal models used by her lab, to her collaborations with clinicians, physicists, palaeontologists and engineers. Hers is also a long term strategy which acknowledges that the quest for a solution is one that is beyond immediate grasp but is nonetheless of vital importance.
Current predictions using Genome Wide Association Studies (GWAS) suggest that there could be up to 100 genes involved in osteoarthritis (OA). It is very unlikely that any one gene has a significant effect on its own. Coupled with the fact that it is a disease borne of environmental and genetic components, it will possibly never be reduced to one single cause. This makes it a particularly challenging area to work in and perhaps explains why researchers have struggled to find a cure.
“While our research will not lead directly to clinical benefits in the short term, it should form the basis for applied work in the future, identifying new targets against which drugs can be developed. Given how many people are affected and are likely to be affected by joint deterioration in their lifetime, identifying potentially ‘druggable’ genetic targets is of paramount importance.” - Dr Chrissy Hammond
Mutating zebrafish genes to observe joint development
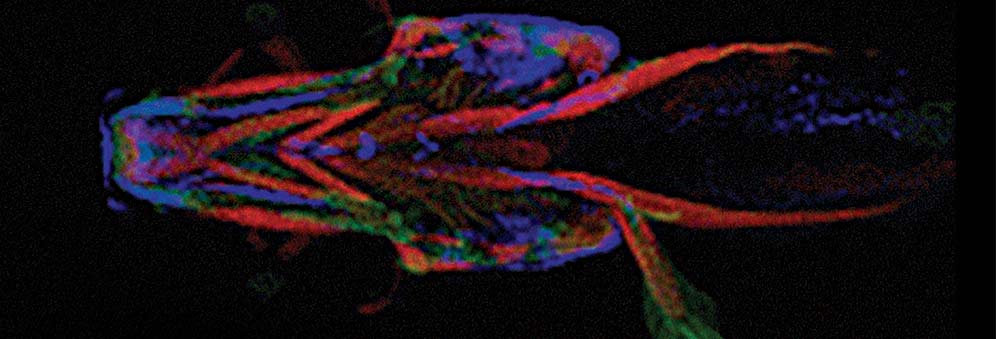
The core component of Dr Hammond’s lab, part Biochemistry, part Physiology and Pharmacology, involves working out the precise role of each of the 100 genes associated with OA. This is done by modifying each gene in the zebrafish, which owing to its fast rate of development, is an ideal model for mapping the development of an age-related disease.
By mutating each gene, researchers can observe the impact each mutation has on joint development and deterioration – how it affects the shape of cartilage and the cells within it, and whether it leads to premature breakdown of cartilage.
“Although the process is slow, the potential is huge,” explains Dr Hammond. “We now think we have identified about ten per cent of the genes through GWAS, but we’re still only scratching the surface.”
Mapping genes against human development
The second strand of Dr Hammond’s research involves identifying “the unknown quantity” – the genes that might be involved but which haven’t been picked up by GWAS. Using DNA fingerprinting, mutations are randomly dropped into the DNA of the zebrafish. Live imaging enables researchers to compare what happens in the mutant fish and the non-mutant fish, and whether the genes are actively involved in bone deterioration. This process of elimination is gradually narrowing down the possibilities of which genes can be mapped against human development.
“If we can identify the genes responsible for causing skeletal problems in zebrafish that are reminiscent of osteoarthritis, we’ll then be able to use these zebrafish to study the biological changes underlying progression of the human disease.” - Dr Chrissy Hammond.
Dr Hammond’s lab has already identified that changes to the CHST11 gene leads to premature cartilage degeneration in zebrafish. The same gene has recently been identified by collaborators working with human patients. This finding could explain why certain people, but not everyone, see beneficial effects from taking a chondroitin and glutamine sulphate – a supplement that is popular among people with joint conditions but which clinical trials have not provided conclusive evidence to support.
By identifying the genetic cause of each problem Dr Hammond’s research is allowing her to establish which treatments certain people are more likely to respond to, increasing the likelihood of success in treating joint conditions.
Cross-discipline collaboration enables greater insights
The nature of OA and the questions it poses about the genetic and structural vulnerabilities of the human body, also lends itself to many fruitful collaborations across the disciplines. One of the questions Dr Hammond’s lab is interested in is what makes the perfect joint during development? The answer is being sought out with the help of engineers and palaeontologists, whose sophisticated modelling techniques allow Dr Hammond to visualise the impact of how the strains from muscle activity lead to changes to the shape of the joint.
That data only makes sense if researchers know how zebrafish muscle and cartilage actually behave at a material level. For that, Dr Hammond turns to physicists where atomic force microscopy provides the ideal level of detail for such a small animal model. While nano-indentation, courtesy of colleagues in the Dental School, provides a particularly useful tool given that the zebrafish larvae under analysis are just 2mm wide.
The insights yielded by Dr Hammond's research lab are early indicators of success in understanding the complex factors affecting joint deterioration and in finding a solution for the treatment and prevention of osteoarthritis.